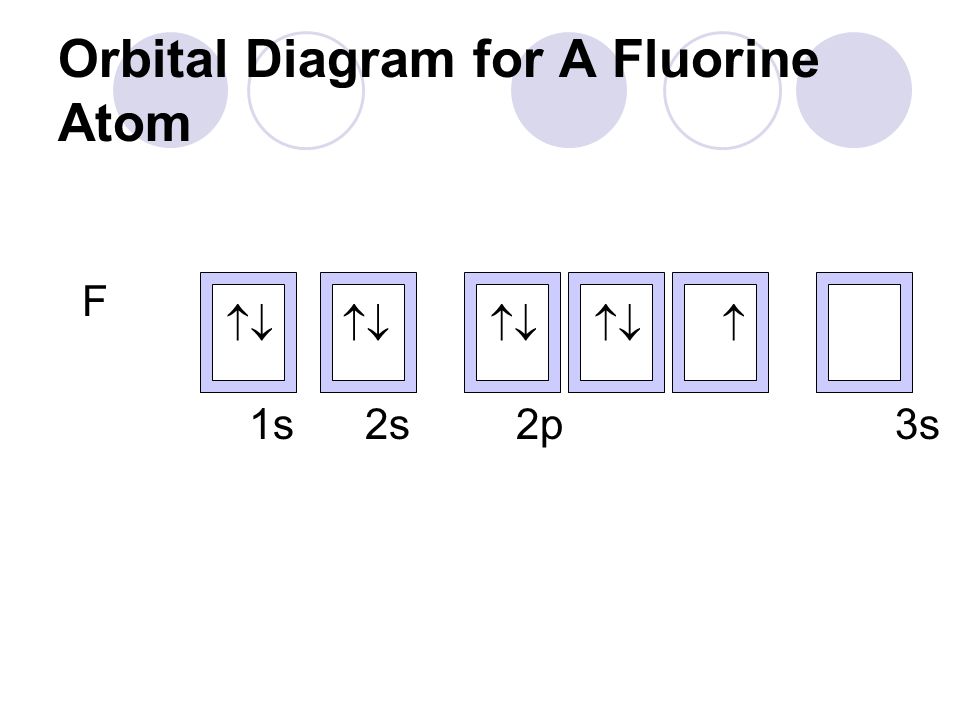
orbital diagram of fluorine
Sapling Hw Ch 116. Atomic energy shells are subdivided into sub-energy levels.

Orbital Configurations Ck 12 Foundation
Orbital diagram for phosphorus Electron configuration of phosphorus in the excited state.
. In an internal combustion engine the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some. The Magnesium orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital and the remaining two electrons in the 3s orbital. The ground state electron configuration of silicon is 1s 2 2s 2 2p 6 3s 2 3p 2.
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram. S orbital can hold maximum of 2 electrons and P orbital can hold maximum of 6 electrons. On some screens the V for vanadium element 23 may look a bit like a Y.
The fluorine atom wants to be more stable like the neon atom by accepting an electron. This is called quantum jump. The s-block element is sodium.
Atoms can jump from one orbital to another in an excited state. 33000000 ft where it merges into the solar wind. The terms atomic orbital and molecular orbital were introduced by Robert S.
This layer is mainly composed of extremely low densities of hydrogen helium. Valence electrons- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic nucleus. This is called quantum jump.
For example because fluorine has an energetically favorable EA and a large energy barrier to ionization IE it is much easier to form fluorine anions than cations. These sub-energy levels are also called orbital. Electron configuration of hydrogen through orbital.
The orbital diagram for Magnesium is drawn with 4 orbitals. Mulliken in 1932 to mean. The upper limit of the atmosphere.
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular. Milankovitch cycles describe the collective effects of changes in the Earths movements on its climate over thousands of years. The exosphere is the outermost layer of Earths atmosphere ie.
1s is the closest and lowest energy orbital to the nucleus. The program ran from October 24 1957 to December 10 1963. An internal combustion engine ICE or IC engine is a heat engine in which the combustion of a fuel occurs with an oxidizer usually air in a combustion chamber that is an integral part of the working fluid flow circuit.
They tend to fill this shell by gaining an electron from other atoms making them negatively-charged ions. This is called quantum jump. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same.
The lithium atom donates an electron of its last orbit to the fluorine atom and LiF forms compounds through ionic bonds. The orbitals are 1s 2s 2p and 3s. Then play a game to test your ideas.
The ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. To write the orbital diagram of sodiumNa you have to do the electron configuration of sodium. For a diatomic molecule the atomic orbitals of one atom are shown on the left and those of the other atom are shown on the right.
Group 17 elements including fluorine and chlorine have seven electrons in their outermost shells. Halogen elements are fluorineF chlorineCl bromineBr iodineI and astatineAt. We already know that the p-subshell has three orbitals.
Metallic properties including conductivity and malleability the ability to be formed into sheets depend on having electrons that. The water cycle also known as the hydrologic cycle or the hydrological cycle is a biogeochemical cycle that describes the continuous movement of water on above and below the surface of the EarthThe mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice fresh water saline water salt water and atmospheric. What is the orbital diagram for Magnesium Mg.
In most cases electrons fill the lower-energy orbitals first followed by the next higher energy orbital until it is full and so on. This isnt a mistake but an effect of converting my original diagram into a lower quality gif image for efficient web use. The electrons mass is approximately 11836th that of the proton.
The Boeing X-20 Dyna-Soar Dynamic Soarer was a United States Air Force USAF program to develop a spaceplane that could be used for a variety of military missions including aerial reconnaissance bombing space rescue satellite maintenance and as a space interceptor to sabotage enemy satellites. Substitute two of the H labels with ethyl and fluorine to construct the more stable conformation of cis-1-ethyl-2-fluorocyclohexane. Yes fluorine is a smaller atom than Li so atoms in the 2s orbital are closer to the nucleus.
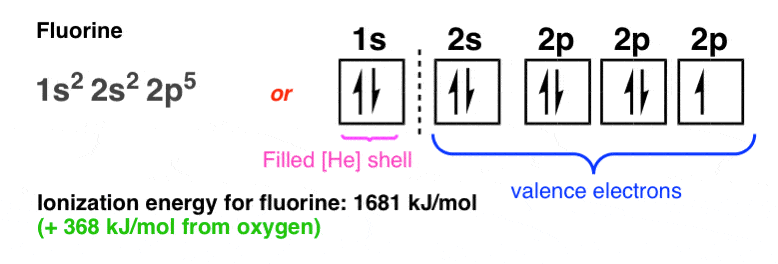
The ground-state electron configuration of fluorine is 1s 2 2s 2 2p. Orbital Diagram Fluorine Electron configuration of fluorine in the excited state. Build an atom out of protons neutrons and electrons and see how the element charge and mass change.
Orbital diagram for siliconSi Silicon excited state electron configuration. The hydrogen atom combines with the carbon fluorine chlorine oxygen and silicon atoms to. The electron is a subatomic particle denoted by the symbol e or β whose electric charge is negative one elementary charge.
Create the atomic orbital diagram for nitrogen Sapling Hw Ch 115. Atoms can jump from one orbital to another orbital in the excited state. The term was coined and named after Serbian geophysicist and astronomer Milutin MilankovićIn the 1920s he hypothesized that variations in eccentricity axial tilt and precession combined to result in cyclical variations in the intra-annual and latitudinal.
To create an orbital diagram of an atom you first need to know Hunds principle and Paulis exclusion principle. It extends from the thermopause at the top of the thermosphere at an altitude of about 700 km above sea level to about 10000 km 6200 mi. Relating orbital filling to the Periodic Table.
Term symbols with LS coupling. In biology and biochemistry the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reactionThe active site consists of amino acid residues that form temporary bonds with the substrate binding site and residues that catalyse a reaction of that substrate catalytic site. For light atoms the spinorbit interaction or coupling is small so that the total orbital angular momentum L and total spin S are good quantum numbersThe interaction between L and S is known as LS coupling RussellSaunders coupling named after Henry Norris Russell and Frederick Albert Saunders who described this in 1925 or spin-orbit.
Which has been discussed in detail above. In chemistry a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a moleculeThis function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. Atoms can jump from one orbital to another in an excited state.
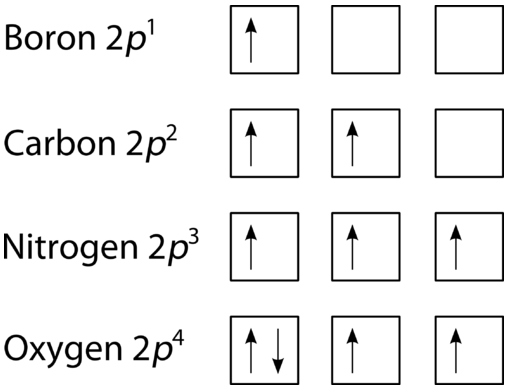
Although the active site occupies only 1020 of the volume of an. Looking at the orbital diagram of oxygen. Or the electronic configuration of Carbon is 1s 2 2s 2 2p 2 since it contains a total of 6 electrons the first two-electron will go in the 1s orbital the next two in the 2s orbital and the remaining two electrons will go into the 2p orbital.
The electron configuration of all the elements can be done through the orbital diagram. For example fluorine could be written as 1s 2 2s 2 2p 5 and neon as 1s 2 2s 2 2p 6. Electrons belong to the first generation of the lepton particle family and are generally thought to be elementary particles because they have no known components or substructure.
Electron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of electrons like the orbital diagram. Use the numbering provided on the ring. Sodium reacts with.
How many σ and π bonds are in this molecule cover left side.

Pictorial Molecular Orbital Theory Chemistry Libretexts

Solved Construct The Orbital Diagram Of The F Ion A Chegg Com
Review Of Atomic Orbitals Master Organic Chemistry
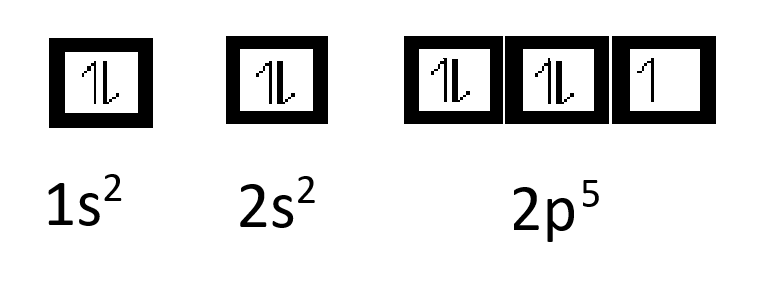
Write The Full Orbital Diagram For Each Element A N B F Quizlet

Fluorine F
What Is The Molecular Orbital Diagram Of O2 And F2 Quora

Electron Configuration For Fluorine F

Electron Configuration And Orbital Diagrams Ppt Video Online Download
The Atom Ppt Video Online Download
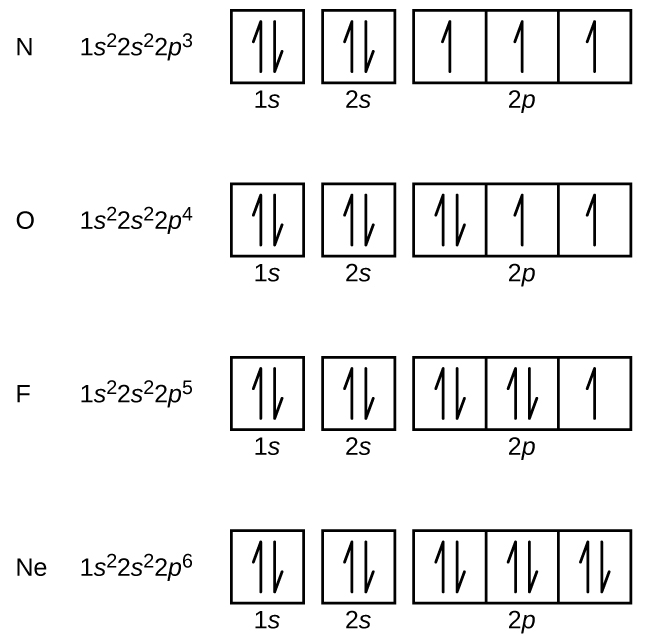
M7q7 Electron Configurations Orbital Box Notation Chem 103 104 Resource Book
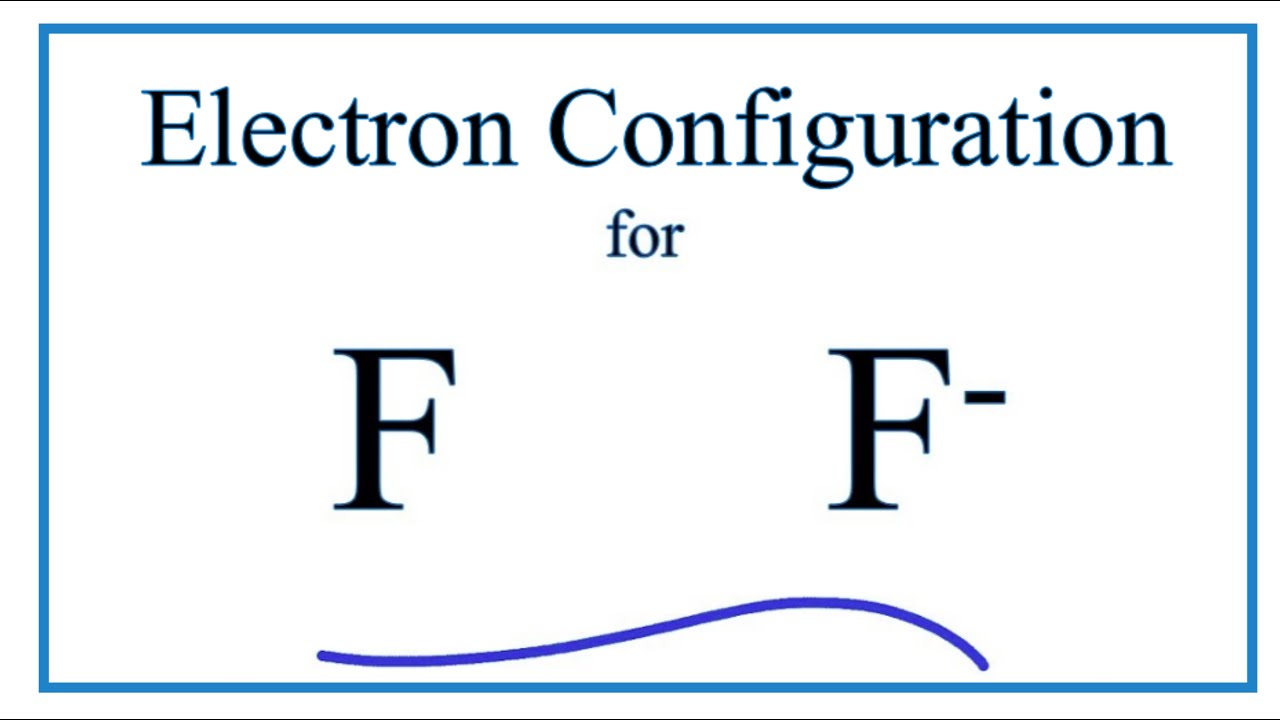
How To Write The Orbital Diagram Fluorine F Youtube
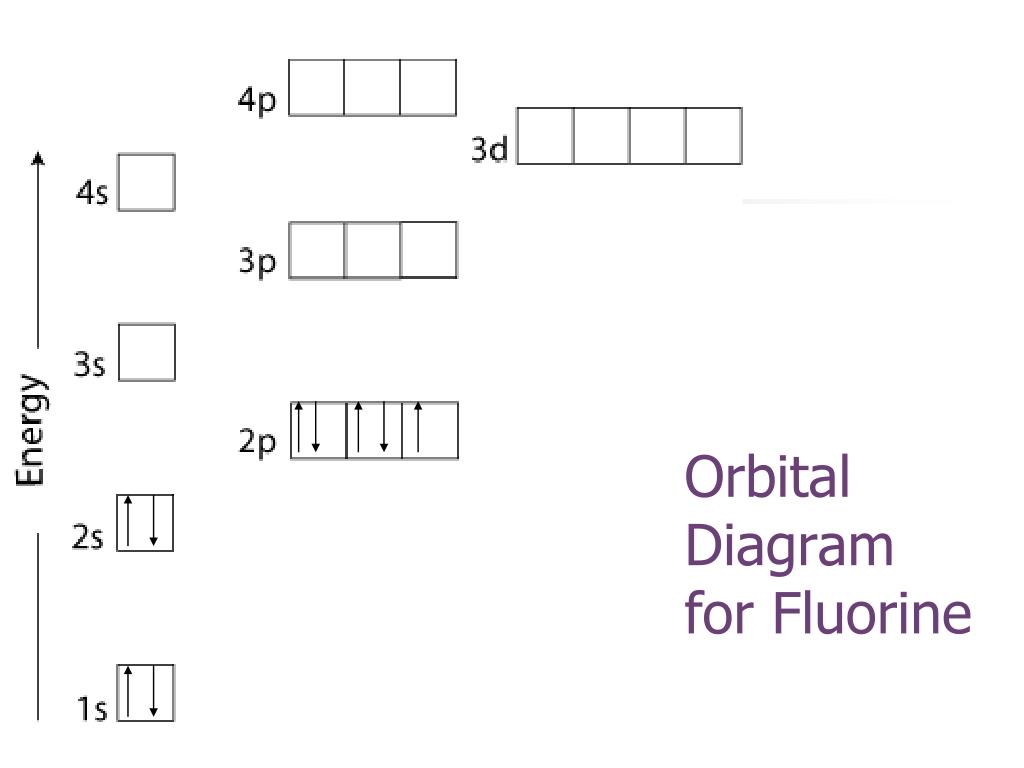
Ppt Electron Configuration Powerpoint Presentation Free Download Id 2644003

Write The Full Orbital Diagram For Fluorine Homework Study Com

9 3 Molecular Orbital Theory Chemistry Libretexts
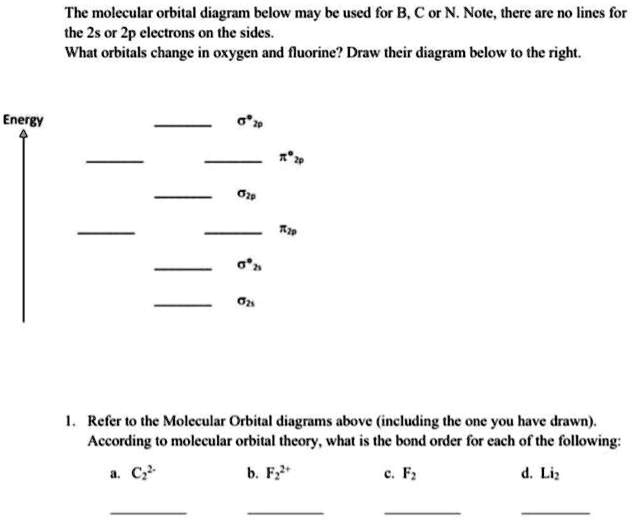
Solved The Molecular Orbital Diagram Below May Be Used For B C Or N Nole There Are No Lines For The 2s Or Zp Electrons On The Sides What Orbitals Change In

Molecular Orbital Diagram Of O2 F2 And Ne2 Molecules Youtube

5 17 Hund S Rule And Orbital Filling Diagrams Chemistry Libretexts